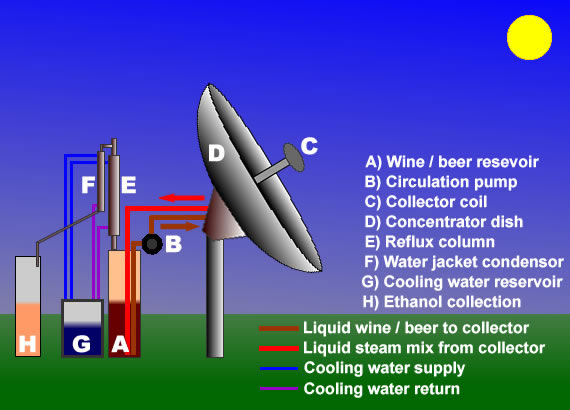
Solar distillation of ethanol alcohol for fuelUsing sunshine to make fuel! It's not moonshine, it's sunshine.Read below about how we use a parabolic dish and a reflux still to produce fuel alcohol.ALCOHOL FUEL PRODUCER PERMIT #AFP-CO-15040
Here's how it works: The reservoir tank (A) is filled with wine, beer, or other fermented liquid. It is pumped by the circulation pump (B) through the solar collector coil (C) which is heated by solar energy focused on it by the concentrator dish (D). Hot liquid and steam is injected back into the bottom of the reservoir tank which brings it to a boil (A). Steam rises into the reflux column (E). The temperature gradient in the reflux column fractions the rising steam into water and ethanol. Water vapor condenses and falls down the column while ethanol vapor rises to the top and passes into the water jacket condenser, (F) where it condenses back into liquid and is collected in the fuel canister (H). The water jacket condenser requires a constant flow of cool water pumped from the cooling water reservoir (G) to condense the ethanol vapors back into liquid. Cooling water also is circulated at a regulated rate through the cooling coils on the reflux column to keep the still head temperature from rising too high and allowing water into the condenser. Hot water exiting the condenser and cooling coils can be used to heat the next batch for distilling. This way, we use maximize solar energy.
There is controversy surrounding ethanol and whether it contributes a net gain or loss in the sustainable energy equation. One of the main drawbacks with ethanol fuel is that it takes energy to manufacture - first in the cultivation and harvesting of sugar crops, and again in the distillation process. Another drawback is that the cultivation of crops to make fuel could compete for resources needed to grow food. But consider this: - regardless of where it fits into the sustainability equation, ethanol is one of the only liquid fuels that can be easily made by the average person. If the gas stations run dry, not to worry! If you can procure sugar and yeast; with a well crafted still you can make fuel to power your car, motorcycle or lawnmower. It might not be very feasible on a large scale, but on a small scale, this process empowers the individual tremendously. With the exception of Italy and New Zealand, personal manufacture of ethanol is illegal. Fortunately, in the United States a person can apply for an alcohol fuel producer permit, which provides a legal avenue for citizens to make their own ethanol for fuel. The catch: you must surrender the property where your still is located to spontaneous government inspection. This is a big deterrent for many people. The people we talked with at the TTB were professional, friendly, and to the point. They issued our permit in less than a month. It seemed to move things along that we had a facility dedicated to this type of research and had everything setup and ready to go.
Reference Information: Heat properties of Ethanol vs. Water
Energy content of various fuels http://peswiki.com/index.php/Directory:Butanol Energy Content in Btu/gallon The following table and descriptions were taken directly from the following link where full explanations of this table and references also can be found. Most of the information is more specific to butanol, a bio fuel produced by fermenting bio mass with bacteria rather than yeast.
Energy content and effects on fuel economy Air-fuel ratio Specific energy Heat of vaporization
|